pH: It's pHundamental
By: Bill Rufenacht | Technical Specialist, Dairy Connection Inc.
Why does pH matter in making fermented dairy products?
In the manufacture of cultured dairy products (cheese, yogurt, kefir, etc.) starter cultures are responsible for the task of converting lactose (milk sugar) to lactic acid. The amount of lactic acid produced plays a key role in creating the flavors and textures desired in finished products. Since the bacteria inoculated in milk operate on a microscopic level – it is difficult to know what’s happening during the make process by simply observing. Measuring pH is an effective way to tell how starter cultures are performing during the process of making a finished cultured product.
pH is:
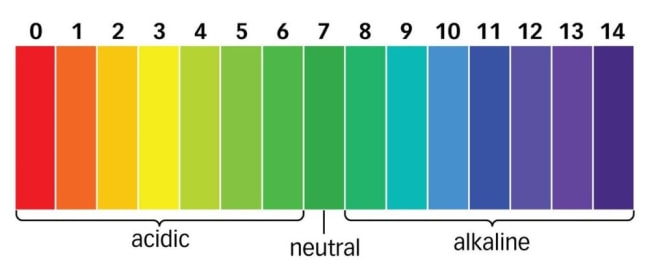
Most simply, pH is a scale (pH 0 to pH 14) indicating the acidity or alkalinity level of a solution. More accurately, pH is a measure of the strength or intensity of an acidic reaction. Acidity depends upon the presence of H+ ion (hydrogen ions) and alkalinity depends on the presence of the OH- ion (hydroxide ions). The proportion of H+ to OH- ions determines the pH of a system. When the ions are present in equal amounts the reaction is neutral. The pH at this neutral point is designated as pH 7.0.
More precisely, pH is (the science):
Acids:
When H+ ions exceed the OH- ions, the reaction is acidic, and the pH value falls below 7.0. Acidic reactions range from just below 7.0 to 0.0 depending on the strength of the reaction. The stronger the reaction, the lower the value. Acids range from pH 0.0 to pH 6.99.
Bases:
If OH- ions exceed the H+ ions in number, the reaction is considered alkaline. Alkaline substances are also called bases. These pH values range from just over 7.0 to 14.0, depending upon the degree of alkalinity. The higher the value, the greater the strength of the reaction. So, bases (alkaline reactions) range from pH 7.01 to pH 14.0.
For those interested:
The pH scale runs from 0.0 to 14.0. In mathematical terms, 1 / pH = log (H+). Because a log scale is involved, a one-unit change in pH represents a ten-fold change in acidity. A pH of 4.0 is: ten times more acidic than a pH of 5.0; 100 times more acidic than a pH of 6.0; and 1000 times more acidic than pH 7. It becomes evident that small changes in pH can have a large impact on your products.
A word about temperature:
Temperature will effect the pH of a solution. As temperature increases the ability of water to ionize increases (creating more hydrogen ions) and pH will decrease. Many (most) pH meters you will encounter will have a temperature probe built in to compensate for that change.
About Your Milk:
The pH of fresh milk from a healthy animal is normally given as 6.6-6.7, slightly acidic. This number can vary depending on the type of animal, the overall health of the animal, feed and water sources.
Why should I monitor pH in my process?
Knowing the pH of a product during manufacture is important because it gives the ability to make informed decisions about what’s happening in your process. Knowing the pH value:
- Provides target values for important steps in the process (Process Control!)
- Provides a basis for knowing when an enzyme (a compound that speeds a biological process) is functioning at the optimum rate (saving time and money!)
- Provides insight into general quality, texture, flavor, and overall microbial status of the fermentation (happy bacteria, happy product, happy customers, happy cheesemaker!)
- Helps to determine whether disease-causing bacteria may grow in your product (safe food!!!!)
- Helps us as technical service at Dairy Connection or Get Culture understand what is going on with your process, when you call with a question or problem.
Your finished products:
Typical pH would normally be in the following range:
- Mature surface ripened varieties (Brie, Limburger, near the end of Best Use By) – 6.5 or higher
- Swiss or Fresh Mozz – 5.4
- Muenster, Gouda – 5.30
- Colby, Monterey – 5.20
- Cheddar – 5.10
- Cream Cheese – 4.9
- Feta – 4.6
- Chevre – 4.6 or lower
- Yogurt – 4.5 or lower
And in the end …
pH is a valuable tool to use in monitoring the overall well-being of your starter culture, and the progress of your milk becoming a finished product.
Have a question about pH?
Ask us in the comments, or give us a call! Our technical staff is always happy to help.
Next time…
Basic use and care of pH meters.
Recent Blog Posts
-
Featured Product: YoMix© ABY-2C Yogurt Culture
By: Bill Rufenacht | Technical Specialist, Dairy Connection Inc.YoMix© ABY-2C is a yogurt start …2nd Apr 2024 -
Featured Product: Bioprotective Cultures
By: Bill Rufenacht | Technical Specialist, Dairy Connection Inc.Let’s take a look at bioprotective …15th Feb 2024 -
Introducing: CONNECT U
If you've ever spent time composing a mission statement, you know that the process can be an exce …12th Oct 2023